Combined protocol for SAM/MAM treatment: The ComPAS study
By Jeanette Bailey, Rachel Chase, Marko Kerac, André Briend, Mark Manary, Charles Opondo, Maureen Gallagher and Anna Kim
Jeanette Bailey is the Project Director for ComPAS, based at the International Rescue Committee (IRC) in New York. She has an MSc in Public Health Nutrition and is undertaking her PhD at the London School of Hygiene & Tropical Medicine. She has more than 10 years’ experience of working in nutrition programmes in humanitarian contexts.
Dr Rachel Chase received her PhD from the Department of International Health at Johns Hopkins Bloomberg School of Public Health. She currently works as a qualitative and quantitative data analyst for universities and non-governmental organisations.
Dr Marko Kerac is a lecturer in Public Health Nutrition and the course director for the MSc in Nutrition for Global Health at the London School of Hygiene & Tropical Medicine (LSHTM). He is a medical doctor with a PhD in nutrition and many years of experience in leading research on malnutrition in developing countries.
Dr André Briend is an Adjunct Professor in the Department of International Health, University of Tampere and Affiliated Professor in Child Nutrition at the Department of Nutrition, Exercise and Sports at the University of Copenhagen. He is a medical doctor with a PhD in nutrition and has more than 30 years of experience in research in paediatric nutrition in developing countries.
Dr Mark Manary is the Helene B. Roberson Professor of Paediatrics at the Washington University School of Medicine in St. Louis. He is a medical doctor with many years of experience leading research on the treatment of acute malnutrition.
Dr. Charles Opondo is a Research Fellow at the LSHTM, and a Researcher in Statistics and Epidemiology at the University of Oxford. He is a pharmacist with an MSc and PhD in Medical Statistics from the LSHTM.
Maureen Gallagher is the Senior Nutrition & Health Advisor for Action Against Hunger U.S., based in New York. She is a public health specialist with an MSc in Social Policy and Planning, specialising in health policy. She has been working in nutrition programming for the last 15 years in Niger, East Timor, Uganda, Chad, DRC, Burma, Sudan and Nigeria
Anna Kim is a Senior Health Communications and Advocacy Officer at the IRC in New York. She has an MA in International Relations and Journalism from New York University.
Special thanks to the donors who funded this work: USAID’s Office of U.S. Foreign Disaster Assistance (OFDA); particularly Mark Phelan, Erin Boyd, Sonia Walia and Jonathan Hamrell, and the Children’s Investment Fund Foundation (CIFF); particularly Claire Harbron.
Thanks to MSF-France and MSF-Spain for sharing data and to IRC, ACF-USA, ACF-UK and the London School of Hygiene & Tropical Medicine colleagues for their collaboration and input during the development of this work, particularly Angeline Grant, Bethany Marron, Lara Ho, Silke Pietzsch, Ruwan Ratnayake, Viddah Owino, Bijoy Sarker, Sophie Woodhead, Saul Guerrero, Amy Mayberry, Kerstin Hanson, Nuria Salse, Candela LaNusse, Stien Gijsel and Severine Frison.
Thanks to WFP and UNICEF for their support and guidance to ComPAS, particularly Saskia de Pee, Britta Schumacher, Lynnda Kiess, Dolores Rio, Diane Holland, Grainne Moloney and Tewoldeberha Daniel.
A more detailed version of this article will be prepared for publication in a peer-reviewed journal.
Location: Chad, Kenya, Yemen, Pakistan, South Sudan.
What we know: Different protocols, products and service systems are used to treat severe and moderate acute malnutrition, which complicates management of the conditions.
What this article adds: Stage 1 of the three-year Combined Protocol for Acute Malnutrition Study (ComPAS) retrospectively analysed treatment data (growth, energy requirements) from acutely malnourished children to develop (by expert committee) a simplified MUAC-based dosing chart to treat both SAM and MAM (Combined Protocol). The study found that growth trends in MUAC mirror those of proportional weight gain during treatment. Rates of gain in MUAC and weight slow with increasing MUAC and as they do, proportional energy needs decrease. Total energy needs of 95% of all children with a MUAC <125mm can be met with 1,000 kcals/day. Given this, a Combined Protocol is proposed that admits children with MUAC<125mm and/or oedema and treats as follows: MUAC <115mm - 2 sachets RUTF/d; MUAC 115mm- <125mm -1 sachet RUTF/d). The next phase of ComPAS aims to examine the effectiveness and cost-effectiveness of this simplified protocol.
The Combined Protocol for Acute Malnutrition Study (ComPAS) aims to simplify and unify the treatment of uncomplicated severe and moderate acute malnutrition (SAM/MAM) for children aged 6-59 months into one protocol in order to improve the global coverage, quality, continuity of care and cost-effectiveness of acute malnutrition treatment in resource-constrained settings. Building on a number of studies (see Box 1), the Combined Protocol proposes to use only one product (ready-to-use therapeutic food (RUTF)), at doses tested to optimise growth and minimise cost at each stage of treatment. Admission, progress and discharge will be assessed using mid upper arm circumference (MUAC) and oedema only. It is hypothesized that this approach will eliminate the need for separate products/infrastructure/administrative procedures for MAM treatment; enable earlier treatment of cases before deterioration into more costly SAM treatment; enable better continuity of care; and lead to more positive community perceptions of the programme.
ComPAS began in October 2014 and completed its first stage of secondary data analysis in January 2016. The second stage will consist of a multi-country cluster randomised trial in two countries and is expected to be completed by December 31, 2017. It is guided by a scientific committee of global experts in paediatrics and nutrition and comprised of partnerships between the International Rescue Committee (IRC), Action Against Hunger-USA (AAH-USA), Action Against Hunger-UK (AAH-UK) and the London School of Hygiene and Tropical Medicine (LSHTM). It is funded by USAID’s Office of U.S. Foreign Disaster Assistance (OFDA) and Children’s Investment Fund Foundation (CIFF).
Stage 1 of ComPAS retrospectively analysed treatment data from acutely malnourished children aged 6-59 months to assess growth trends and energy requirements and develop a simplified dosing chart based on MUAC. Preliminary findings are summarised in this article; a more detailed final analysis is planned for future peer-reviewed publication.
Box 1: What is known? Research underpinning ComPAS
The current SAM treatment protocol bases RUTF dosage on 175-200 kcal/kg/day until children are discharged as cured. However, research indicates food intake drops (Ashworth, 1969) and growth rate (weight and MUAC-based) slows (Goossens et al, 2012) towards the end of treatment. Similar cured/death/defaulter results have been found where RUTF dosage was reduced at the end of SAM treatment (Cosgrove et al, 2012); this observation is being further explored through ACF research 1. A recent trial in Sierra Leone suggests that an integrated SAM/MAM programme using RUTF had more favourable outcomes on coverage and is an acceptable alternative to standard treatment (Maust et al, 2015).
Objectives
The objectives of Stage 1 of ComPAS were to:
1) Assess rate of growth and
a. Calculate energy requirements for observed growth in children recuperating in Outpatient Therapeutic Programmes (OTPs) and Supplementary Feeding Programmes (SFPs);
b. Evaluate differences in the response to treatment according to geography, age, anthropometric status on admission, and treatment outcome;
2) Calculate energy requirements by MUAC category; and
3) Propose a physiologically appropriate dosage table based on MUAC that could be used in a simplified and unified protocol.
Method
A secondary analysis of data from children recovering from SAM in OTP and from MAM in SFP programmes was used to evaluate growth trends in response to treatment, using registration book/patient card data from the following programmes and countries:
- International Rescue Committee (IRC): Chad, Kenya, Yemen.
- Action Against Hunger-USA (AAH-USA): Pakistan.
- Médecins Sans Frontières-France (MSF-France): South Sudan.
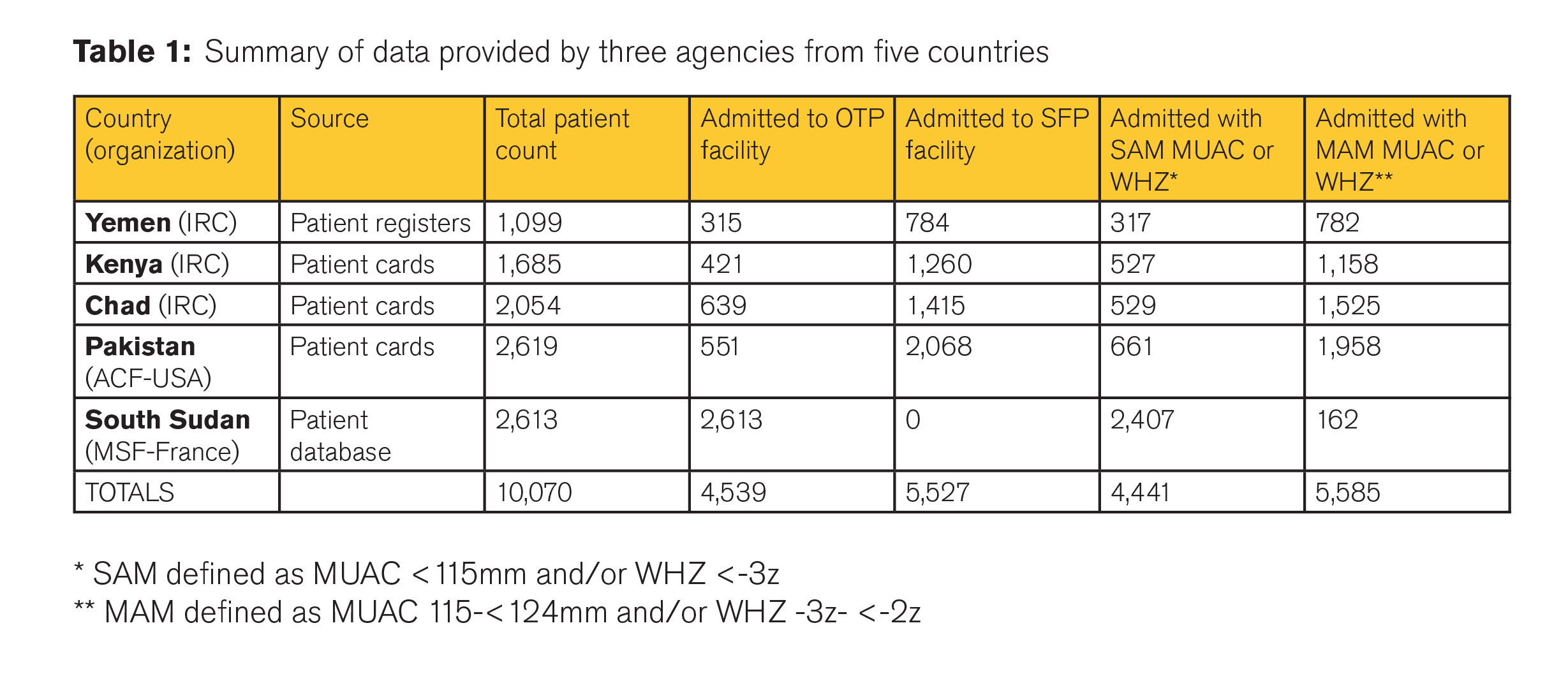
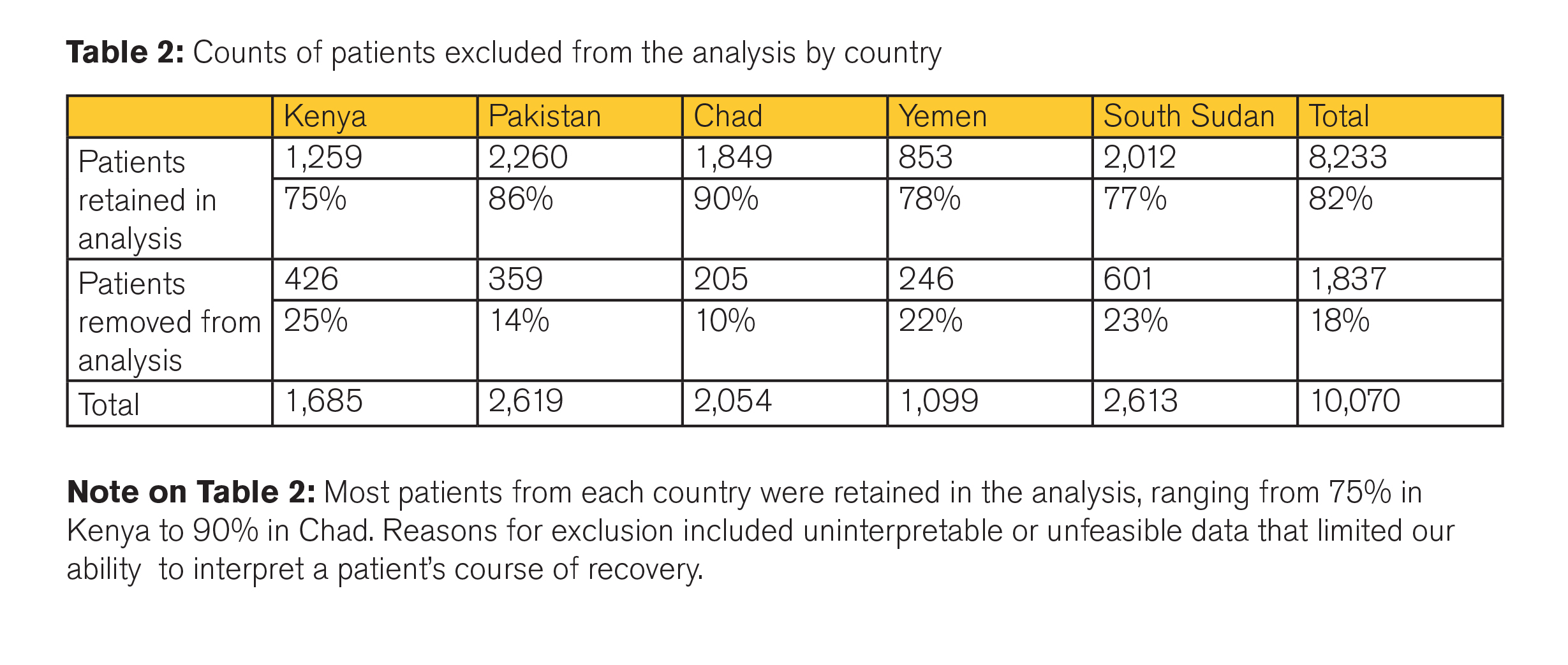
Data from 10,070 acutely malnourished children between the ages of 6 and 59 months were available (see Table 1); 8,233 were retained for analysis (Table 2). Most patients from each country were retained in the analysis, ranging from 75% in Kenya to 90% in Chad. Reasons for exclusion included uninterpretable or unfeasible data that limited interpretation of individual course of recovery.
To balance the contributions of each of the five countries in the analysis when using local polynomial estimation (described below), 1,300 visits were randomly selected from each country for a total of 6,500 visits (from, at most, 2,798 children) in a sub-sample used for the visual analysis of growth. This visual analysis of the local polynomial estimation of growth by MUAC at last visit guided the decision to assess energy needs over ranges of MUACs at last visit in meaningful but theoretical groups.
Method for objective 1: Assess rate of MUAC and proportional weight gain and energy requirements of children and evaluate differences in the response to treatment
Using local polynomial smoothing, we visually assessed the relationship between:
(1) One-week MUAC growth (mm); and
(2) Proportional weight gain (g/kg/day) compared to last-measured MUAC among children discharged as “recovered”.
One-week MUAC growth was calculated as the difference between two MUAC measurements when a child’s visits are one week apart. When a child’s visits were recorded as being two weeks apart, the difference between the first and second visit MUAC is divided by two to estimate weekly MUAC growth. Proportional weight gain was calculated as the weight gain (in grams) divided by weight at last visit (in kg) divided by number of days since the last visit prior to the reference visit (hereafter simply referred to as the “last visit”).
Visual assessment provided initial estimates of optimal knot placement to perform linear spline regression. Knots were placed to mark the edges of spans of MUAC measurements for which MUAC is changing at a near constant rate and where large changes in MUAC growth occur. Nonlinear least squares estimation (via the nl command in Stata 13.1) was used to optimize knot placement after initial visual assessment. Excluded from the analysis were observations with MUAC measurements below 100mm and above 135mm (ranges in which the local polynomial becomes less stable), observations marking extreme MUAC changes (greater than 15mm in one week), and observations from children who were discharged with any result other than "recovered". To further simplify caloric need calculations, a step function was calculated to break at the previously identified knots. Knot locations were considered candidate indicators for dosage recalculation.
This analysis was performed for all data together (unweighted), by region (Africa and Asia), by country, and by age group. Except when compared by country, reported values and figures reflect results from the
n=6,500 sub-sample described above.
Method for objective 2: Calculate energy requirements by MUAC category
Daily energy needs were estimated as:
Current weight (in kg) * resting energy needs (82 kcal/kg) + weight gain (in grams) * energy costs of weight gain (5 kcal/g) (FAO, 2001)
Changes in energy needs were calculated for each child as kcal/day as per the above formula and as kcal/kg weight. Both values are reported by MUAC at last visit. As an example, consider a child who weighed 7.1 kg, had a MUAC of 116mm on their 5th visit to the clinic, and weighed 7.4 kg on their 6th visit 7 days later. This means that the child gained, on average, 42.9 g in weight per day between visits. To estimate the child’s daily energy needs to achieve that growth, we calculate:
7.1 kg * 82 kcal/kg + 42.9 g * 5 kcal/g = 796 kcal. Because the child’s MUAC was 116mm at their 5th visit, this energy need estimate is reported along with other children’s calculated energy needs who had a MUAC of 116mm at their prior visit.
Method for objective 3: MUAC dosage table
An expert scientific committee reviewed the results of objectives 1 and 2 and proposed a MUAC/RUTF dosage table that would cover the total energy needs of >95% of children aged 6-59 months with a MUAC <115mm, and half the energy needs of >95% of children aged 6-59 months with a MUAC 115-<125mm.
Results
Growth trends in MUAC mirror those of proportional weight gain and rates of MUAC and weight gain slow with increasing MUAC.
Amongst children with similar MUACs at a given visit, MUAC and proportional weight gain show roughly the same growth trend. Proportional rate of growth tends to be lower for children with higher MUACs, and rates of growth appear to plateau over ranges of MUACs (see Figure 1).
Figure 1: Growth trends in MUAC and weight
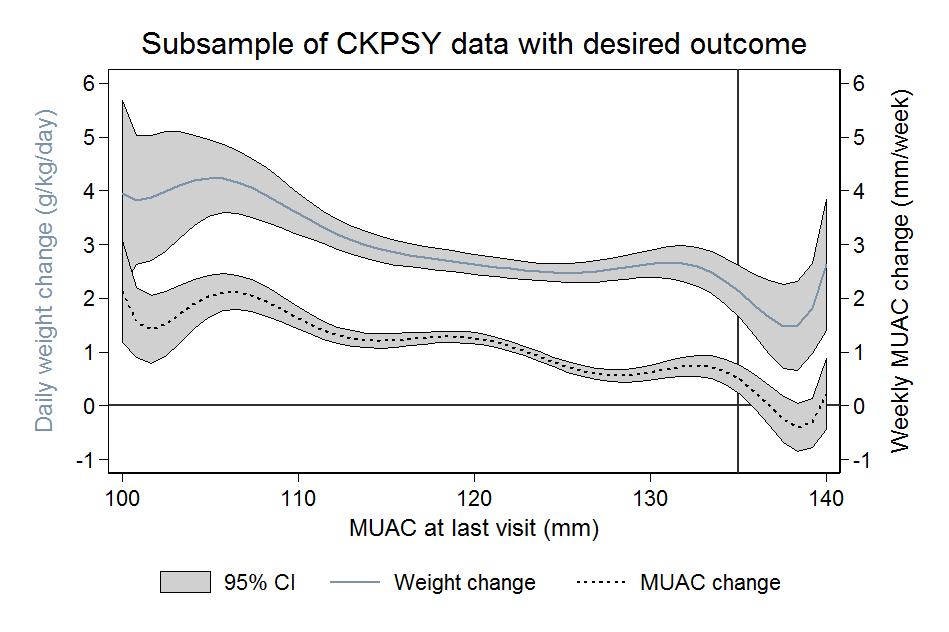
No significant difference was observed in growth trends across MUAC at last visit by age group (under two years and over two years) or by stunting status (greater/equal to or less than height for age (HAZ) <-2 z-scores).
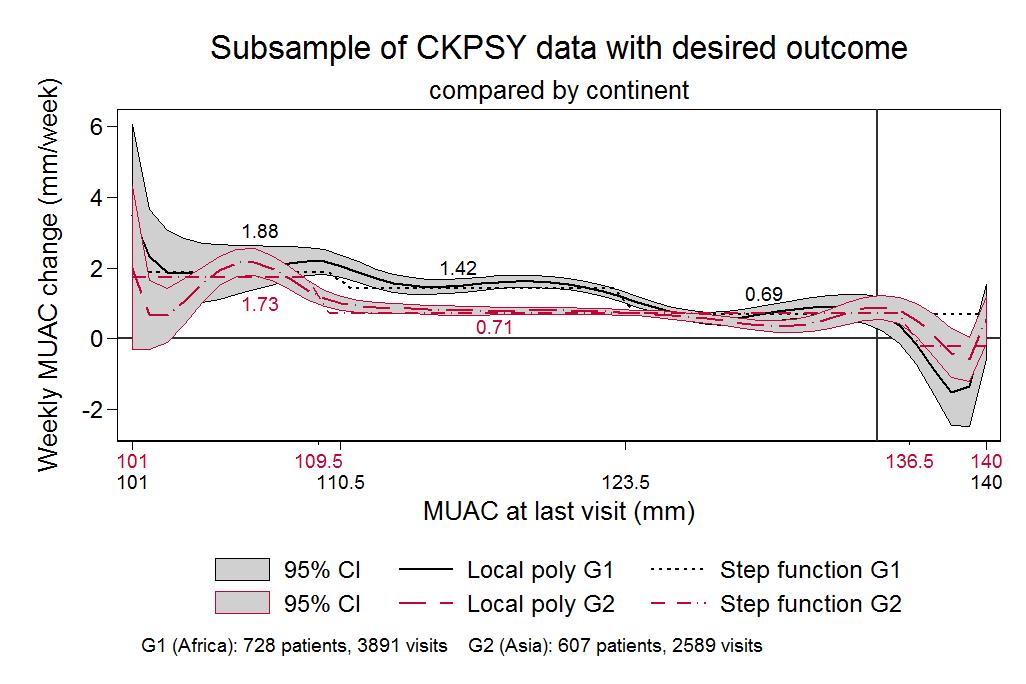
When comparing children enrolled in programmes in the three African countries (South Sudan, Chad and Kenya) and children enrolled in programmes in the two Asian countries (Yemen and Pakistan), a notable difference in weekly MUAC gain is observed between 111mm and 123mm MUAC at last visit (1.4 mm vs 0.7 mm). New plateaus in MUAC gain might be seen at 115mm and 126mm among children in Africa, whereas only one new plateau appears to form among children in Asia over MUAC at last visit, starting at around a MUAC of 110mm (see Figure 2).
Figure 2: Growth trends in MUAC and weight in Asia and Africa
As the rates of MUAC and weight gain slow, proportional energy needs decrease.
Comparisons of MUAC and proportional weight gain to MUAC at last visit indicate that average proportional energy needs (that is, energy needed per kg of weight to achieve observed growth) follow a similar step-down pattern (see Figure 3 online), although total energy needs increase due to greater total body weight. Among children with MUAC <125mm, the children with the greatest energy needs to achieve observed growth are those with the lowest MUACs; approximately 150-160 kcal/kg/day would be enough to support the growth observed in 95% of visits among children with the lowest MUAC. In these data, energy needs never exceed 190 kcal/kg/day (see Figure 3).
Figure 3: Comparisons of MUAC and proportional weight gain to MUAC at last visit
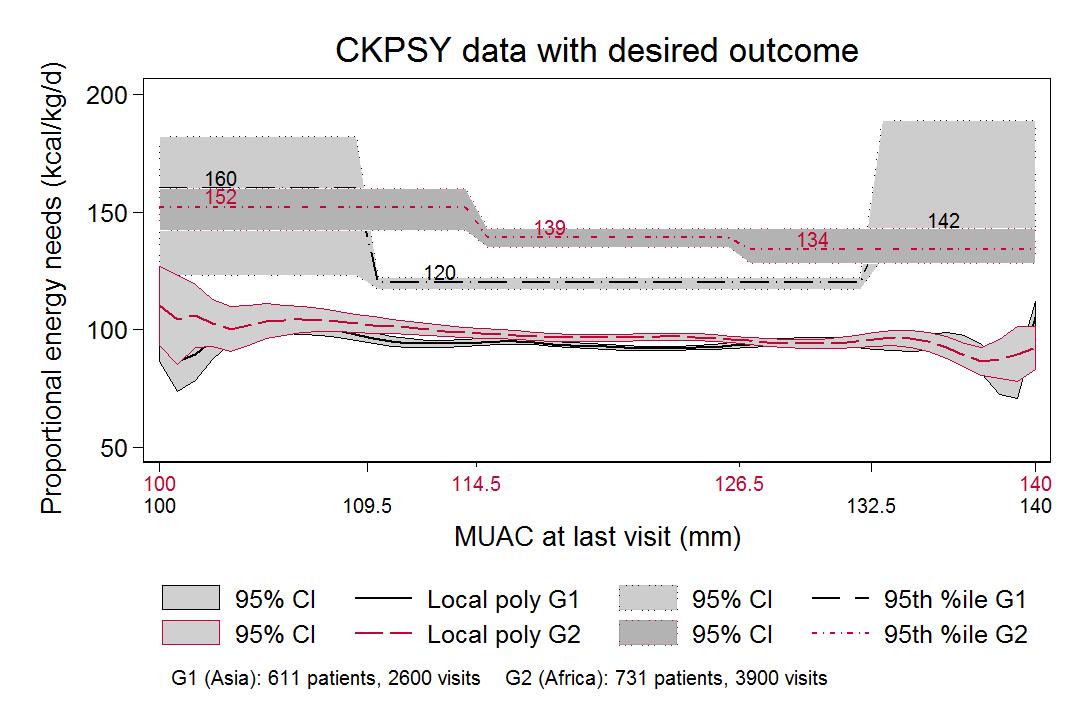
Total energy needs of 95% of all children with a MUAC <125mm can be met with 1,000 kilocalories a day.
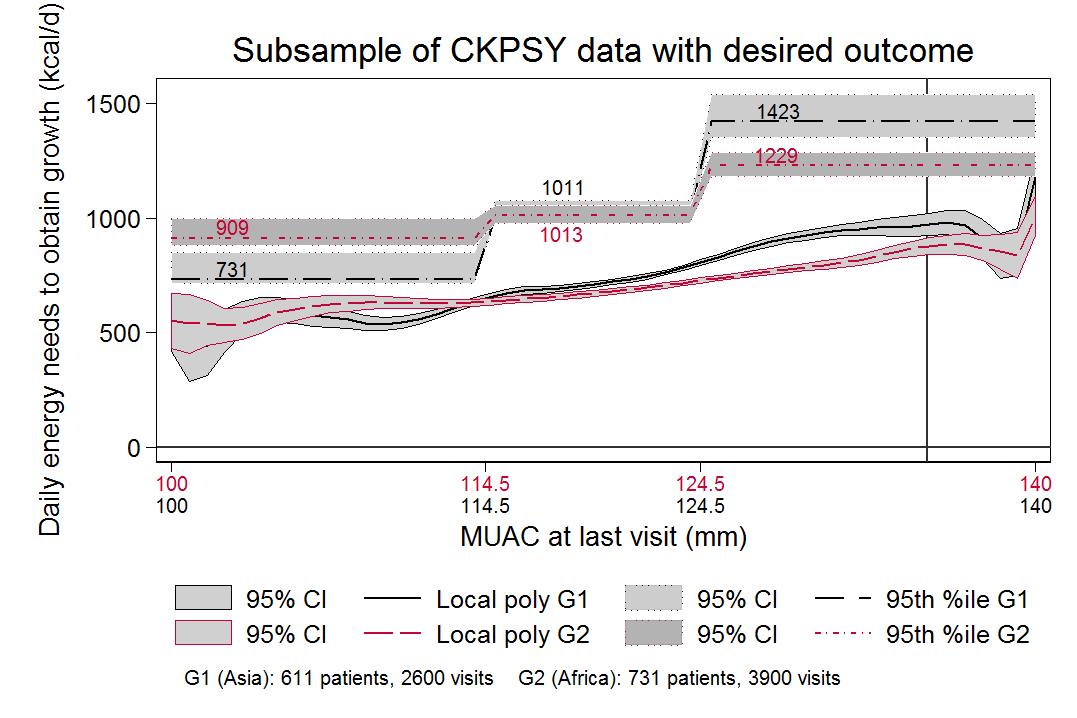
The 95th percentile line indicates the number of kilocalories that would be sufficient to cover the total energy needs of 95% of children with 100mm ≤ MUAC < 115mm, 115mm ≤ MUAC < 125mm, or 125mm ≤ MUAC ≤ 140mm. Minor differences were observed between estimated energy needs for children in Africa and Asia when MUAC was below 115mm (909 kcal in Africa vs 731 kcal in Asia), and no practical difference when 115mm ≤ MUAC < 125mm (1,013 in Africa, 1,011 in Asia) (see Figure 4).
Figure 4: Daily energy needs to cover growth by MUAC at last visit
MUAC dosage table
The ComPAS expert panel met to review these results from January 26-27 2016. They agreed that MUAC was a suitable alternative to weight to determine the dosage of RUTF as children progress through treatment. The Combined Protocol will admit all children who have a MUAC<125mm and/or oedema, and treat them according to the following simplified dosage protocol:
- Children with a MUAC <115mm or oedema (+) to receive two sachets of RUTF per day (1,000 kcal);
- Children with a MUAC 115mm- <125mm to receive one sachet of RUTF per day (500 kcal).
This protocol remains in line with globally accepted practice, with children recovering from SAM receiving enough therapeutic food to cover their total energy needs (as a replacement for the family diet), whereas children with MAM receive a food supplement to complement their family diet. Most SFP protocols provide 500-550 kcal/day of ready-to-use supplementary food (RUSF). The advantage of using one product is simplicity (currently procurement of RUTF and RUSF involves separate UN agencies) and physiological appropriateness, considering acute malnutrition on a continuum of severity rather than as separate SAM/MAM conditions.
When tested in a theoretical comparison (Table 3), the protocol performs as designed (i.e. meets the total energy needs for >95% of children with a MUAC <115mm, and meets approximately half the energy needs for >95% of children with a MUAC 115-<125mm) for all major sub-divisions of children (by sex, country, continent, weight and admission type). Of note:
- At 95% of visits, children with MUAC < 115mm in the specified category have 100% of their energy needs covered by two sachets of RUTF (1,000 kcal) per day. Two categories of children fall slightly short of this goal: children aged 25 months or more (93%) and children who weigh 8.0kg or more (83%). Even in these two groups, only at 5% of visits do children have less than 96% of their caloric needs covered by two sachets of RUTF per day.
- At 95% of visits, children with a MUAC of 115mm -< 125mm have 50% or more of their total energy needs met by the provision of one RUTF sachet (500 kcal) per day. In both Asia and Africa, we estimate that 49% of total energy needs or more are met at 95% of visits. Children aged 25 months or over and children who weigh 8.0kg or more again fall short of this goal, but not by a significant amount: only 5% of children aged 25 months or more and children who weigh 8.0kg or more have 40% and 42% respectively (or fewer) of their energy needs covered to achieve observed growth. However, the provision of RUTF 500kcal per day matches the current global MAM management practice of providing 500 kcal of RUSF per day, so the same gap exists with the existing SFP protocol. The nutrient compositions of RUTF and RUSF are not exactly the same (RUTF has more dairy and RUSF has a higher content of macro-minerals, important for linear growth), but RUTF has been proven effective in the treatment of MAM (Maust et al, 2015).
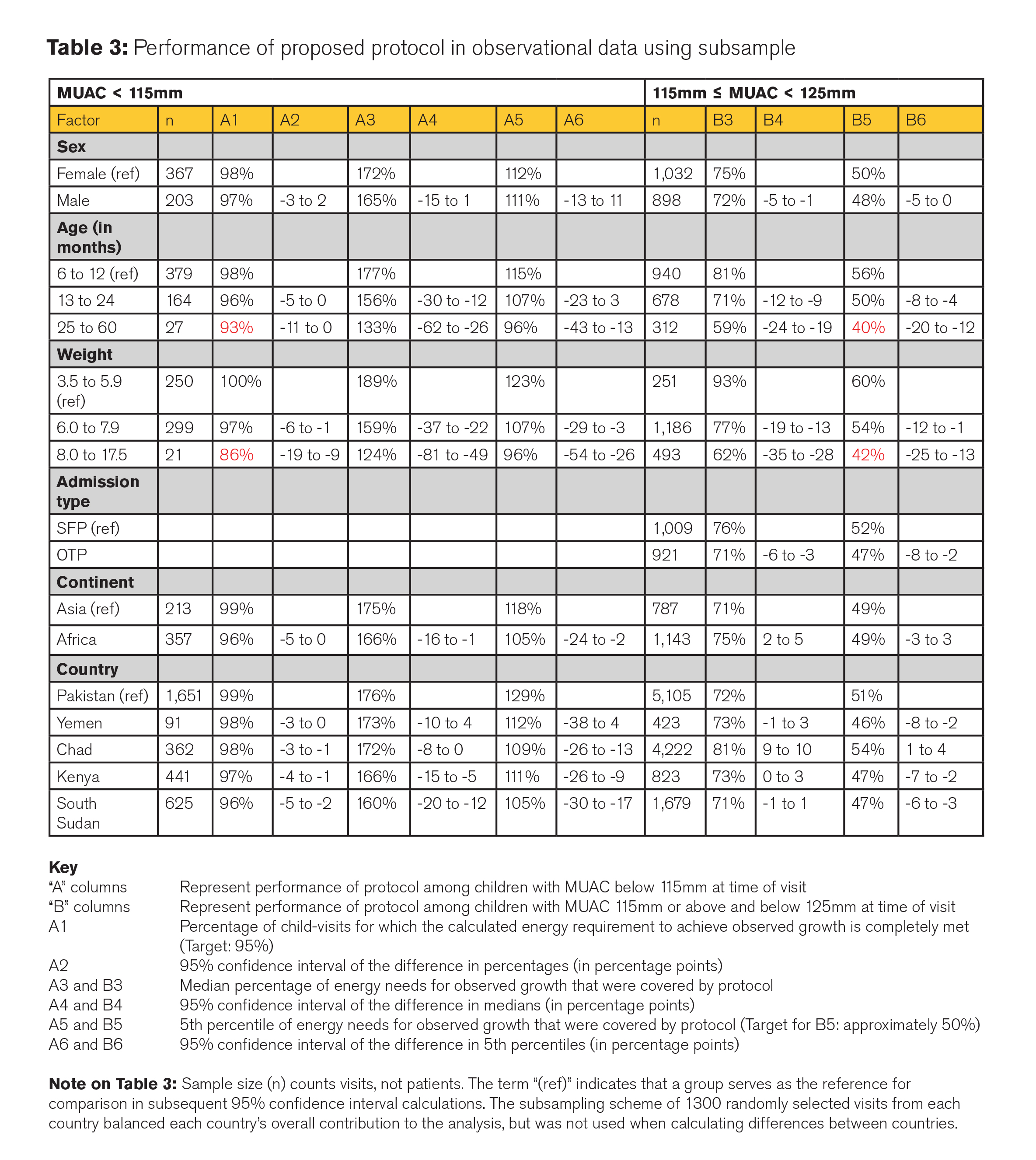
Overall, 97% of children with MUAC < 115mm in the observational data would have their total energy requirements to achieve observed growth met or exceeded by the proposed protocol. The median percentage of their energy needs covered would be 170% (i.e., they would receive 70% more calories than needed to achieve observed growth), and 95% of children with MUAC < 115mm would have at least 111% of their energy needs covered by the protocol.
Consistent with the design, 95% of children with 115mm- < 125mm would have 49% or more of their energy needs covered by the proposed protocol, with a median of 74% of their energy needs covered. In both Africa and Asia, this simplified dosage protocol meets or exceeds the vast majority of children’s energy needs when children have MUAC < 115mm and is aligned with the current practice of SFP programmes of providing at least 50% of children’s energy needs when children have a MUAC between 115-<125mm.
Conclusion
This study considered the rate of weight and MUAC gain and energy needs of children with acute malnutrition as defined by MUAC status. Our findings concluded that two 92g sachets of RUTF (1,000 kcal) meet the total energy requirements for >95% of children with a MUAC<115mm, and one 92g sachet of RUTF (500 kcal) meets half the energy requirements for >95% of children with a MUAC of 115-<125mm, and serves to simplify and streamline the treatment to be tested in a combined protocol.
The next phase of ComPAS aims to examine the effectiveness and cost-effectiveness of this simplified protocol. Stage 2 will pilot the combined protocol in a cluster randomised controlled non-inferiority trial in two countries to assess the effectiveness of the combined protocol against the standard protocol (OTP + SFP) in terms of recovery (with enrolment and discharge based on MUAC), coverage, length of stay, and average daily weight gain and weekly MUAC gains. A comprehensive cost-effectiveness analysis will be included as part of the field trial.
For more information, contact: Jeanette Bailey, email: Jeanette.bailey@rescue.org
References
Ashworth A. Growth rates in children recovering from protein-calorie malnutrition. British Journal of Nutrition. (1969), 23,835
Goossens S, Bekele Y, Yun O, Harczi G, Ouannes M, Shepherd S (2012). Mid-Upper Arm Circumference Based Nutrition Programming: Evidence for a New Approach in Regions with a High Burden of Acute Malnutrition. PLoS ONE 7(11).
Cosgrove N, Earland J, James P, Rozet A, Grossiord M, Salpeteur C. Qualitative review of an alternative treatment of SAM in Myanmar. F. Exch. [Internet]. 2012;42:6. Available from: www.ennonline.net/fex/42/qualitative
Maust A, Koroma A, Abla C, Molokwu N, Ryan K, Singh L, Manary M. Severe and Moderate Acute Malnutrition Can Be Successfully Managed with an Integrated Protocol in Sierra Leone. Journal of Nutrition. 2015.
FAO. Food and Agriculture Organization. Human energy requirements: Report of a Joint FAO/WHO/UNU Expert Consultation. FAO Food Nutr. Tech. Rep. Ser. [Internet]. 2001;0:96. Available from: www.fao.org/docrep/007/y5686e/y5686e08.htm
1
Modeling an Alternative Nutrition Protocol Generalizable for Outpatient (MANGO) research study in Burkina Faso in 2016-2017. www.isrctn.com/ISRCTN50039021


