Implementation of a field study of body composition among infants and young children in sub-Saharan Africa
By Ezekiel Mupere, John Mukisa, Lynnth Turyagyenda, Peace Aber, Luke S Uebelhoer, Celine Bourdon, Robert Bandsma, Emmanuel Chimwezi, Jonathan C Wells, Wieger Voskuijl, Judd L Walson, James A Berkley and Christina Lancioni
Ezekiel Mupere is a Senior Lecturer and Chair of the Department of Paediatrics at the College of Health Sciences, Makerere University, Kampala, Uganda and Co-Principal Investigator of the Kampala CHAIN study.
John Mukisa is a medical officer, epidemiologist and biostatistician who served as study coordinator for the Kampala CHAIN site at Mulago Hospital until February 2019.
Lynnth Turyagyenda is the head nutritionist for the Kampala CHAIN Team.
Peace Aber is the lead data manager and statistician for the Kampala CHAIN Team.
Luke S Uebelhoer is a Senior Research Associate in the Department of Pediatrics at Oregon Health & Science University and manager of Kampala CHAIN operations and immunological research.
Celine Bourdon is a research data analyst in the Division of Gastroenterology, Hepatology and Nutrition at the Hospital for Sick Children, Toronto, Canada and oversees data management for the Malawi CHAIN site.
Robert Bandsma is an Assistant Professor of Paediatrics and staff gastroenterologist at the Hospital for Sick Children, Toronto, Canada and manager of clinical assessments, sample collection and patient care at the Malawi CHAIN site.
Emmanuel Chimwezi is a data manager at the University of Malawi College of Medicine, Blantyre. He oversees data collection, input, cleaning and management at the Malawi CHAIN site.
Jonathan C Wells is a Professor of Anthropology and Pediatric Nutrition at the Childhood Nutrition Research Centre at the UCL Institute of Child Health in London, UK. Dr. Wells is an expert in the application of techniques to study body composition in young children and serves as an advisor to the CHAIN study.
Wieger Voskuijl is a general paediatrician at Emma Children’s Hospital at the Amsterdam University Medical Center, clinical researcher at the Amsterdam Institute for Global Health and Development, and lead Principal Investigator of the Malawi CHAIN study.
Judd L Walson is a Professor of Global Health, Allergy and Infectious Diseases, Pediatrics and Epidemiology at the University of Washington and co-Principal Investigator of the CHAIN Network.
James A Berkley is a Professor of Paediatric Infection Diseases, Senior Clinical Research Fellow and Group Head and Consultant Physician at the Centre for Tropical Medicine and Global Health in the Nuffield Department of Medicine at the University of Oxford. He is co-Principal Investigator of the CHAIN Network.
Christina Lancioni is an Assistant Professor and Pediatric Infectious Disease specialist in the Department of Pediatrics at Oregon Health & Science University and co-Principal Investigator of the Kampala CHAIN study.
We would like to thank the Ugandan and Malawian children and care providers who gave their time and dedication to participate in this project. We would also like to thank Dr. Victor Owino for his early assistance on this project, as well as our funder, the Bill & Melinda Gates Foundation.
An important question regarding continuum of acute malnutrition treatment concerns the differences and similarities between severely and moderately malnourished children that contribute to poor outcomes. Assessment of body composition is an evolving area to help improve risk identification and inform targeted care (Eds).
Location: Malawi and Uganda
What we know: There are several techniques available for direct measurement of body composition; the gold standard is isotope dilution technique. Bio-electrical impedance analysis (BIA) is affected by hydration status and has not been validated in low- and middle-income countries (LMIC).
What this article adds: As part of the Childhood Acute Illness & Nutrition (CHAIN) Network cohort study across nine sites in Asia and Africa, body composition of severe acute malnutrition (SAM), moderate acute malnutrition (MAM) and normally nourished hospitalised children were assessed in two sites (Malawi and Uganda) at hospital discharge and 90 days post-discharge. Isotope dilution was used to establish and validate prediction models of body composition for measures of anthropometry, BIA and skinfold thickness. The study found BIA and skinfold thickness could be practically implemented in a multi-site study of infants and children in LMIC. However, results could not be validated due to difficulties in application of the isotope dilution protocol in young and undernourished children. Revision, adaptation and field testing of this gold standard is necessary to resolve this. Results regarding body composition by nutrition status will be available in forthcoming peer review publication.
Use of body composition to assess recovery from disease and undernutrition
Body composition reflects nutritional intakes, losses and needs over time. Unlike conventional anthropometry, techniques to measure body composition can quantify tissue losses by analysing two distinct body compartments: fat mass (FM) and fat-free mass (FFM). During periods of undernutrition, loss of FFM and FM varies with increasing duration of undernutrition. Loss of FFM has been associated with decreased survival, worse clinical outcomes, and poor quality of life in some diseases, including an increased rate of infections, complications, hospitalisations, length of hospital stay and recovery period (Pichard et al., 2004; Pirlich et al., 2006; Cetano et al., 2016; Thibault, Genton and Pichard, 2012). In addition, data suggest that body composition in children remains salient in monitoring growth and development, progression of disease, and response to treatments as shown in studies of children recovering from critical illness and Sickle Cell disease (Eke et al., 2015; Zamberlan et al., 2019). Despite these observations, there are little published data from low- and middle- income countries (LMIC) regarding whether the outcomes of mortality and growth recovery in undernourished children can be ascribed to changes in FM and FFM.
Conventional anthropometry includes measurements of weight, length/height and mid upper-arm-circumference (MUAC) to generate age- and sex-specific z-scores used to monitor growth throughout childhood. Conventional anthropometry has been widely adopted to identify undernourished children, classify the severity of undernutrition, and monitor recovery and growth trajectory among children undergoing nutritional rehabilitation. Despite its simplicity and effectiveness in identifying children most at risk of poor outcomes, conventional anthropometry cannot assess differences in body composition among undernourished children; specifically, their FM and FFM.
In clinical practice, nutritional recovery of young children is typically gauged by short-term weight gain. However, an increase in weight does not necessarily reflect an increase in FFM as weight gain following severe illness has been shown to be secondary to gains in FM rather than FFM. Despite accounting for height and weight, Body Mass Index (BMI) also does not accurately reflect FFM, as individuals with similar BMI can have significant variations in FFM. There is mounting evidence that quantification of body composition can be used to gauge illness severity and predict outcomes of childhood illnesses. For instance, children treated for malignancies have significantly lower body cell mass and higher FM compared to healthy, age-matched children (Murphy et al., 2010). A study of critically ill children supports the hypothesis that measurements of body composition, including Phase Angle (an indicator of cellular membrane integrity and correlate of FFM), can predict outcomes from severe illness (Zamberlan et al., 2019). We predict that quantitative assessment of body composition among young children in LMIC can be used to identify those children most at risk of poor outcomes following an acute illness and to gauge recovery during rehabilitation for severe malnutrition.
Techniques for measurement of body composition in low- and middle- income countries
Conventional anthropometry is relatively quick and can be executed by health care workers with minimal training. However, this method only provides an indirect estimate of body composition that depends on the biological inter-relationships among tissues and their expected distribution among normal individuals (Roche, 1996). As a result, such indirect methods have larger predictive errors that are dependent on the disease type that is being examined. For example, Papathakis et al. (2005) compared body composition as measured by isotope dilution, bioimpedance spectroscopy and anthropometry in asymptomatic HIV-infected and -uninfected breastfeeding mothers. Using isotope dilution as the reference standard, BMI and MUAC were useful predictors of FM in both groups but did not accurately estimate FFM in HIV-infected mothers (Papathakis et al., 2005). Notably, Bila et al. (2016) also reported that isotope dilution was superior to conventional anthropometry in the assessment of body composition in overweight and obese European children.
Several techniques are available for direct measurement of body composition, with isotope dilution using deuterium oxide remaining the gold standard (Roche, 1996). This technique is based on determining total body water (TBW), including both extracellular and intracellular water. During the isotope dilution procedure, a known volume of the inert radionucleotide deuterium oxide (99.9 atom % 2H2O) is ingested. The 2H2O mixes with body water but is largely excluded from body lipid stores, and hence allows the differentiation of FFM (which includes the non-lipid components of adipose tissue) and FM. An equilibrated distribution is reached after 3-5 hours in healthy children, though potentially longer in malnourished children with oedema. Saliva or urine (depending on the analysis technique) is then collected to measure the amount of deuterium in body water above that naturally present (known as the enrichment of body water), which can be used to directly calculate TBW and derive FFM and FM.
BIA permits indirect extrapolation of FFM and FM by analysing a low electric current as it travels easily through the body’s conductive water but is impeded by fat. Differences in electrical conductivities between positive and negative electrodes positioned on distal ends of a subject’s limbs are measured by a BIA machine that reports out resistance, reactance, impedance, and phase angle. These measurements represent an index of the TBW that depends on the overall electrical conducting properties of a body with varying tissue composition. Standardised equations are then applied to calculate predicted FFM and FM for an individual. However, these equations vary by age, maturation state, ethnicity and illness; hence, for accurate results this approach requires the derivation of an appropriate equation for the study population. The phase angle measured by BIA is considered an estimate of cellular membrane integrity and lean tissue mass (Lukaski, 1987). BIA has been extensively used in studies of body composition in healthy subjects due to its ease of application, capacity to rapidly process outputs, and relative low cost. However, BIA measurements depend on hydration status (Lukaski, 1996) and importantly, the equations used to calculate FFM and FM are derived from children in high income settings and have not been well validated in young children in African settings (Wells, 2014). Measurement of various skinfold thicknesses (e.g. triceps, subscapular and other regions) is another widely adopted, non-invasive approach to estimate body composition used in both low- and high- income settings. However, this technique is very operator-dependent, and is difficult to perform in young children; thus, results may not be reproducible. In addition to these limitations, the triceps skinfold varies considerably by sex and can reflect differences in the underlying triceps muscle rather than an actual change in body fatness.
In this article, we describe challenges in the practical implementation of a multi-site study in LMIC comparing the techniques of conventional anthropometry, skinfold thickness, BIA, and isotope dilution to measure body composition. Our population of interest is infants ages 2-23 months who have severe, moderate, or normal nutritional status, and are recovering from hospitalisation.

Integration of direct and indirect measures of body composition in a multi-site paediatricfield study
The Childhood Acute Illness & Nutrition (CHAIN) Network is a cohort study currently being carried out across nine sites in Africa and South Asia, with the purpose of understanding determinates of morbidity and mortality among infants and young children aged 2-23 months following hospitalisation for an acute illness.1 Using MUAC, children are classified at hospital admission as severely acutely malnourished (SAM, including children with nutritional oedema), moderately acutely malnourished (MAM), or normally nourished (NAM). Children are enrolled within 24 hours of their hospitalisation, closely monitored throughout their hospital stay, and then followed-up 45-, 90- and 180-days after discharge. An age-matched reference population of community children is also recruited at each site. The CHAIN Network aims to determine modifiable risk factors for highly vulnerable children. In this network, two sites (Mulago Hospital in Kampala, Uganda and Queen Elizabeth Hospital in Blantyre, Malawi) aimed to: 1) establish whether FFM, FM and phase angle as measured by BIA at hospital discharge are predictors of re-admission or post-discharge mortality; 2) describe the post-discharge changes in FFM, FM and phase angle as measured by BIA 90 days following hospital discharge; and 3) use isotope dilution to establish and validate prediction models of body composition for measures of anthropometric, BIA and skinfold thickness in hospitalised children of different nutrition backgrounds at discharge, day 90 post-discharge and in community children.
Indirect measures of body composition: Challenges and solutions for protocol harmonisation and minimisation of operator-dependent measurements
Skinfold thickness and BIA are operator-dependent measurements and prone to errors due to insufficient training and variation in technique, as well as minor differences in instrument calibration, use, and maintenance. The CHAIN study therefore tackled operator bias by: 1) standardising all equipment (BIA was assessed using Quadscan 4000 (Bodystat), skinfold thickness was performed using a Harpenden Skin Fold Caliper, weight was obtained using SECA 334 and 335 electronic scales, length was measured with the SECA 416 infantometer and MUAC was recorded using UNICEF tape); 2) developing Standardised Operating Procedures (SOPs), which were followed by all stakeholders to guide measurement recording and equipment maintenance; 3) standardising instrument calibration across the two sites at the start of and throughout the study; and 4) organising in-person joint trainings to ensure SOP harmonisation. Even though researchers and nutritionists in both the Ugandan and Malawian teams had previous experience with measuring conventional anthropometry, skinfold thickness and BIA, we found that in-person SOP training was essential to harmonise procedures and obtain precise and accurate measurements. Table 1 presents the post-training quality assessment of anthropometry, BIA, and skinfold thickness measurements performed by the Ugandan and Malawian CHAIN teams. The Technical Error of Measurements (TEM) summarises inter-evaluator performance of the study team personnel as compared to gold standard measurements obtained from a nutritionist with five years of field experience in assessing anthropometry in children (Lynnth Turyagyenda). ‘Relative TEM’ expresses TEM as a percentage of the average value of that variable and these were classified as “acceptable” or “unacceptable” based on established criteria (Perini et al., 2005). In this study, we considered the variables weight, length, head circumference, MUAC, subscapular skinfold thickness, reactance, and resistance, and computed their TEM.
Table 1: Inter-evaluator variations between the Ugandan and Malawian teams as evaluated by Technical Error of Measurements (TEM) and relative TEM
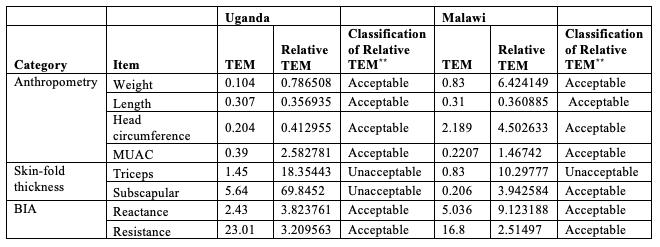
**TEM classification based on the cut-off values by Perini et al. (2005).
The TEM of team members were generally small (less than 10) except for the BIA measurement of resistance. This suggests that the differences between measurements done by CHAIN Network personnel and the gold standard were very small. Relative TEMs were classified as acceptable for both the Ugandan and Malawian teams, except those for skinfold thickness. Additional training was subsequently organised to ensure harmonisation of measures for BIA resistance and skinfold thickness. This resulted in improved quality of measurements for the entire study (data not shown).
Operationalisation of isotope dilution protocol for direct measurement of body composition: Challenges and solutions for protocol implementation
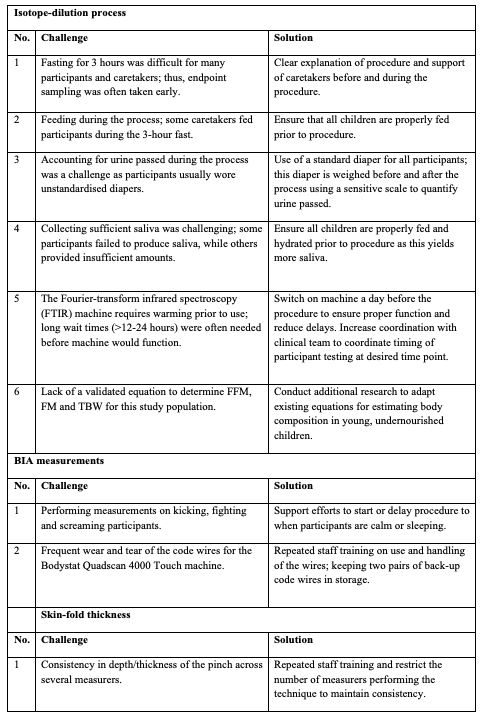
As isotope dilution is a more complex and demanding procedure, it was conducted only by the CHAIN Ugandan team since one of their lead researchers (John Mukisa) had direct experience with performing this procedure in children. For this, the International Atomic Energy Agency (IAEA) protocol was followed (IAEA, 2010) and an experienced consultant facilitated the design, training, implementation, analysis and data interpretation (Ndagire et al., 2018). From the early isotope dilution experiments, we calculated FM (Figure 1) and found that, despite overhead technical guidance, several challenges arose when implementing the protocol among young children (detailed in Table 3). For example, after analysing the first set of samples, it became apparent that results were compromised by: 1) small amounts of oral intake during the three-hour procedure (e.g. breastfeeding to calm an infant); 2) missed collection of outputs (e.g. urine soaked up by diaper); 3) small variations in overall procedure time; or 4) imprecise dosing due to the child not consuming the full volume of isotope mixture.
Figure 1: Fat mass values as calculated by isotope dilution procedure performed at hospital discharge among Ugandan children
Isotope dilution procedure was performed on 121 children of varied nutritional status (SAM, MAM, NAM) at time of hospital discharge. Shown are fat mass values of each child grouped by nutritional category.
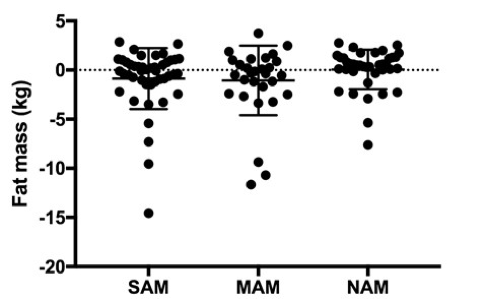 Despite efforts to implement current recommendations for isotope dilution, much of the generated data yielded problematic results: 1) many FM values were negative among children in all nutritional categories; 2) calculated FM did not reflect expected differences between nutritional groups (as was observed when FM was calculated by BIA (Table 2)); 3) isotope dilution was particularly problematic among children with SAM, where the highest variance in values was observed. We also compared the estimated values for FM and FFM at hospital discharge among children with SAM and MAM, as measured by BIA and isotope dilution in the same child (Table 2). Here we noted statistically significant differences in the estimated FM and FFM in both groups of children depending on the applied method, with BIA providing more plausible values. Analysis of body composition of SAM, MAM and normal-weight children, including longitudinal data, will be shared in an upcoming peer-reviewed publication and summarised in a future Field Exchange article.
Despite efforts to implement current recommendations for isotope dilution, much of the generated data yielded problematic results: 1) many FM values were negative among children in all nutritional categories; 2) calculated FM did not reflect expected differences between nutritional groups (as was observed when FM was calculated by BIA (Table 2)); 3) isotope dilution was particularly problematic among children with SAM, where the highest variance in values was observed. We also compared the estimated values for FM and FFM at hospital discharge among children with SAM and MAM, as measured by BIA and isotope dilution in the same child (Table 2). Here we noted statistically significant differences in the estimated FM and FFM in both groups of children depending on the applied method, with BIA providing more plausible values. Analysis of body composition of SAM, MAM and normal-weight children, including longitudinal data, will be shared in an upcoming peer-reviewed publication and summarised in a future Field Exchange article.
Table 2: Variation in estimation of fat mass and fat-free mass as estimated by BIA and isotope dilution
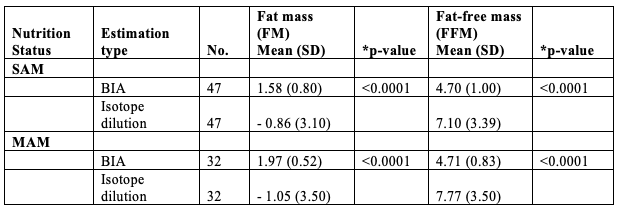
*paired t-test
Many, but not all children, with non-plausible/negative FM as estimated by isotope dilution were observed to have minor protocol deviations. Although the data improved with corrective procedures (detailed in Table 3), it became apparent that the current international protocol for conducting isotope dilution was inadequate to accurately measure body composition in young, undernourished infants in LMIC. A main challenge was to deliver the correct dose of isotope (3 gm in 10 ml as per recommendation) to infants and young children; if a small amount remained unconsumed, calculated TBW was over-estimated. A 1% error in dose (in this case 0.03 gm) produces a 1% error in TBW, and this positive error bias results in variable but systematic over-estimation of TBW and, thus, inaccurate FM and FFM. Technical measurement biases and/or errors were apparent in children of all nutritional backgrounds. Concordantly, we also noted that the isotope-equilibration time appeared to differ among children with varying nutritional status: the greater the level of malnutrition, the longer the isotope needed to equilibrate. Thus, these differences in equilibration dynamics between groups may significantly impact measurement error and variability. Proposed solutions to these and other issues encountered are detailed in Table 3.
Conclusions
Assessment of body composition can provide an in-depth understanding of weight changes in terms of FFM and FM that could allow for earlier and objective management of undernutrition. In this field study, we found that measuring body composition using conventional anthropometry and BIA analysis could be practically implemented in a multi-site study of infants and children in LMIC. However, due to difficulties in implementing current IAEA protocols in infants in our setting, we were unable to validate these indirect measurements against the reference standard of isotope dilution. Our experience has highlighted several issues and solutions that could prompt the revision and adaption of current IAEA protocols. Application of isotope dilution to measure body composition in our young and undernourished target population would require further field testing before a wider implementation.
For more information, please contact For more information, contact: Dr. Ezekiel Mupere, Makerere University, Kampala, Uganda. 256-414-533-531.
Table 3: Challenges in performance of BIA, skin-fold thickness and IAEA isotope-dilution procedures in paediatric population
Endnote
1CHAIN Network http://chainnetwork.org/
References
Adão Perini TLdO, Glauber & dos Santos Ornellas, Juliana & Palha de Oliveira, Fátima. Cálculo do erro técnico de medição em antropometria. Revista Brasileira De Medicina Do Esporte - REV BRAS MED ESPORTE. 2005;11:81-5.
Bila WC, Freitas AE, Galdino AS, Ferriolli E, Pfrimer K, Lamounier JA. Deuterium oxide dilution and body composition in overweight and obese schoolchildren aged 6-9 years. J Pediatr (Rio J) 2016;92:46-52.
Caetano C, Valente A, Oliveira T, Garagarza C. Body Composition and Mortality Predictors in Hemodialysis Patients. J Ren Nutr 2016;26:81-6.
CHAIN Network. at http://chainnetwork.org/.
Eke CB, Chukwu BF, Ikefuna AN, Ezenwosu OU, Emodi IJ. Bioelectric impedance analysis of body composition of children and adolescents with sickle cell anemia in Enugu, Nigeria. Pediatr Hematol Oncol 2015;32:258-68.
International Atomic Energy Agency (IAEA). Introduction to Body Composition Assessment Using the Deuterium Dilution Technique with Analysis of Urine Samples by Isotope Ratio Mass Spectrometry. www.iaea.org/publications2010.
Lukaski HC. Methods for the assessment of human body composition: traditional and new. Am J Clin Nutr 1987;46:537-56.
Lukaski HC. Biological indexes considered in the derivation of the bioelectrical impedance analysis. Am J Clin Nutr 1996;64:397S-404S.
Murphy AJ, White M, Davies PS. Body composition of children with cancer. Am J Clin Nutr 2010;92:55-60.
Ndagire CT, Muyonga JH, Isabirye D, Odur B, Somda SMA, Bukenya R, Andrade JE, Nakimbugwe D. Assessing the reliability of FTIR spectroscopy measurements and validity of bioelectrical impedance analysis as a surrogate measure of body composition among children and adolescents aged 8-19 years attending schools in Kampala, Uganda. BMC Public Health 2018;18:687.
Papathakis PC, Rollins NC, Brown KH, Bennish ML, Van Loan MD. Comparison of isotope dilution with bioimpedance spectroscopy and anthropometry for assessment of body composition in asymptomatic HIV-infected and HIV-uninfected breastfeeding mothers. Am J Clin Nutr 2005;82:538-46.
Pichard C, Kyle UG, Morabia A, Perrier A, Vermeulen B, Unger P. Nutritional assessment: lean body mass depletion at hospital admission is associated with an increased length of stay. Am J Clin Nutr 2004;79:613-8.
Pirlich M, Schutz T, Norman K, Gastell S, Lubke HJ, Bischoff SC, Bolder U, Frieling T, Guldenzoph H, Hahn K, Jauch KW, Schindler K, Stein J, Volkert D, Weimann A, Werner H, Wolf C, Zurcher G, Bauer P, Lochs H. The German hospital malnutrition study. Clin Nutr 2006;25:563-72.
Roche A. Human body composition. Champaign, IL: Human Kinetics Press; 1996.
Thibault R, Genton L, Pichard C. Body composition: why, when and for who? Clin Nutr 2012;31:435-47.
Wells JC. Toward body composition reference data for infants, children, and adolescents. Adv Nutr 2014;5:320S-9S.
Zamberlan P, Feferbaum RAPoP, Doria Filho U, Brunow de Carvalho WFPoP, Figueiredo Delgado AAPoP. Bioelectrical Impedance Phase Angle and Morbidity and Mortality in Critically Ill Children. Nutr Clin Pract 2019;34:163-71.


